Characterizing Exosomes: The Only Way
Since 2005, Andrew Malloy has been in the scientific product space. He is currently Sr. Director of Sales at Unchained Labs, which purchased NanoView Biosciences, where Andrew has worked for the last 5 years. The company developed a unique technology for characterizing extracellular vesicles (or exosomes).
The multiplexed array specifically binds exosomes to a microarray chip; once bound, the signal-enhancing surface allows the exosomes to be imaged down to 40nm, sized, and counted. Up to 5 EV biomarkers can be measured on individual EVs with up to single-molecule sensitivities requiring no sample pre-purification.
Kimera Labs purchased and utilizes this groundbreaking medical equipment.
Mr. Malloy discusses the purification of extracellular vesicles and distinguishing them from lipoproteins in this video.
• Sodar et al. (2016) suggest very high percentage (95%+) of purification techniques tested were not EVs but rather lipoproteins, HDLs, LDLs, and VLDLs.
• Size or density-based purification regimes alone are not enough to isolate EVs only.
• Many particle detection techniques, therefore, mostly measure lipoproteins and not exosomes.
Biologically Active Units (BAU)
At Kimera, we believe that with all the misleading information being communicated in the marketplace, we need to set the record straight, give you the facts about true exosome content and potency, and offer a challenge to anyone looking to see the truth.
Our founder, Duncan Ross, Ph.D., created an independent testing protocol, which provides objective, standardized nanovesicle technology measurements to correct the misleading concept perpetuated in the exosome industry that particle counts correlate with the strength of exosome products.
Dr. Ross realized years ago that utilizing the NanoSight device to simply count particles present in the sample and to state that they are “exosomes”, was an insufficient means to characterize exosomes products.
Part of the manufacturing process is not just “Can you make it?”, but “Can you qualify it?” Kimera has the most advanced and experienced qualification system for exosomes in the world at this time. To confirm the actual content and quality of our exosomes products, we utilize advanced exosome characterization technologies such as ELISA protein analysis, high pressure liquid chromatography (HPLC), RNA sequencing, NanoSight Nano Tracking Analysis, Atomic Force Microscopy, Electron Microscopy and STORM super resolution fluorescence microscopy.
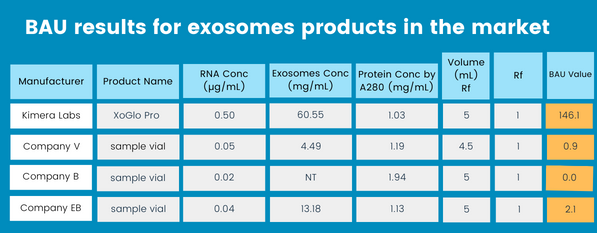
The BAU considers the complexity of the exosomes, not only the particle count, but measures RNA concentration, exosome concentration, total protein concentration, and volume to express an unbiased measurement that can be applied consistently to any product on the market. This number will directly correlate to any exosome’s true potency and, therefore, capability considering Rf=1 for 100% of biological activity. We welcome any other company to bring your products to our lab and be present for the testing process when a head-to-head comparison is made.
After assaying some of the exosome products in the market, these were our findings.
Getting the Facts Straight
At Kimera, this video is the next step in our ongoing effort to get the facts straight, and correct the misleading concept being communicated in the marketplace that particle counts correlate with exosome strength and potency. Our Founder, Duncan Ross PhD, describes the shortcomings of using the Nanosight device as the primary means to quantify a product by counting particles and claiming them to be “exosomes”.
We utilize advanced exosome characterization technologies such as ELISA protein analysis, high pressure liquid chromatography (HPLC), RNA sequencing, NanoSight Nano Tracking Analysis, Atomic Force Microscopy, Electron Microscopy and STORM super resolution fluorescence microscopy to determine actual RNA content, protein concentration, and the actual presence of exosomes in the product. This differentiates Kimera, and our products, from any other company in this space.
